NEET 2025: unlock success Advanced Practice Questions for Mastery in Physical Chemistry

Ace your NEET preparation with a comprehensive set of advanced questions on the critical chapter, “Some Basic Concepts of Chemistry.” Strengthen your understanding and boost your confidence with these practice challenges.
Introduction

With NEET 2025 around the corner, it’s time to enhance your preparation by delving deeper into foundational chapters like “Some Basic Concepts of Chemistry.” This chapter is crucial as it lays the groundwork for more advanced topics in physical chemistry. This article provides an overview of the essential concepts in the chapter, followed by 50 advanced-level practice questions designed to sharpen your skills and help you excel in the exam.
This chapter introduces fundamental principles that are vital for mastering chemistry. Below are the primary topics covered:
- Matter and Its Nature: Understand the states of matter and their distinguishing properties.
- Dalton’s Atomic Theory: Learn the foundational atomic theory and its evolution over time.
- Laws of Chemical Combination: Explore laws like the conservation of mass and definite proportions.
- Atomic and Molecular Masses: Discover the importance of mass in chemical calculations.
- The Mole Concept: Grasp the connection between microscopic and macroscopic worlds.
- Chemical Equations and Stoichiometry: Master the art of balancing equations and determining reaction ratios.
These topics are not only integral to physical chemistry but also influence concepts in organic and inorganic chemistry, making them indispensable for NEET preparation.
Why Practice Advanced-Level Questions?

Challenging practice questions enable deeper comprehension, identify weak areas, and reinforce learning. They also simulate exam-level difficulty, building confidence and preparedness. Below is a curated selection of advanced questions designed for “Some Basic Concepts of Chemistry.”
Advanced Practice Questions success
- Define matter and classify its states with relevant examples.
- Explain Dalton’s atomic theory and its modern limitations.
- Illustrate the law of conservation of mass with a balanced reaction.
- Demonstrate the law of definite proportions using quantitative examples.
- Calculate the molar mass of glucose (C₆H₁₂O₆) and derive its empirical formula.
- Determine the number of moles in 36 grams of water with calculations.
- Predict the moles of sodium chloride formed when 3 moles of sodium react with chlorine gas (include a balanced equation).
- Discuss the mole concept’s relationship with real-world chemical measurements.
- Balance the combustion reaction of propane (C₃H₈) and calculate the enthalpy change given standard enthalpy values.
- Explain stoichiometry and its importance in quantitative chemical analysis.
- Differentiate between empirical and molecular formulas with examples.
- Calculate hydrogen’s percentage composition in water (H₂O).
- Explain Avogadro’s number and its significance in determining molecular quantities.
- Compute the number of molecules in 50 grams of sodium chloride (NaCl).
- Define limiting reactants with a combustion example.
- Describe how temperature affects kinetic energy, referencing kinetic molecular theory.
- Differentiate solutions from colloids with practical examples.
- Analyze how concentration impacts reaction rates using collision theory.
- Define molarity and calculate the molarity of a solution with 58 grams of NaCl in 2 liters of water.
- Discuss colligative properties and their real-world applications, such as boiling point elevation.
- Provide step-by-step instructions for preparing a molarity solution from a solid solute.
- Highlight the role of catalysts in chemical reactions, citing examples like industrial processes.
- Justify the importance of balancing equations for stoichiometric calculations.
- Calculate the volume of gas at STP from moles using oxygen (O₂) as an example.
- Explain ionization energy trends across periods and groups in the periodic table.
- Define oxidation numbers and provide examples involving transition metals.
- Discuss determining empirical formulas through combustion analysis.
- Describe isotopes and their applications, such as in medicine or carbon dating.
- Explain redox reactions with examples of oxidation state changes.
- Discuss calculating molar concentration from moles and volume in practical scenarios.
- Define dynamic equilibrium in reversible reactions and provide real-world examples.
- Explain Le Chatelier’s principle and its effect on equilibrium when temperature changes.
- Calculate the pH of a solution with [H⁺] = 0.001 M and explain its biological significance.
- Define a buffer solution and its role in maintaining pH stability.
- Analyze the effect of temperature on solubility using a salt-water example.
- Discuss Raoult’s Law and its application, such as in antifreeze solutions.
- Explain freezing point depression as a colligative property with examples.
- Evaluate how molecular weight influences boiling point elevation.
- Relate osmotic pressure to biological systems like cell membranes.
- Define specific heat capacity and its role in calorimetry.
- Apply Hess’s Law to calculate enthalpy changes in a chemical reaction.
- Compare endothermic and exothermic reactions with real-world examples.
- Describe how calorimetry measures heat changes in chemical processes.
- Explain reaction mechanisms and their importance in understanding kinetics.
- Analyze energy diagrams for exothermic and endothermic reactions, labeling key components.
- Discuss activation energy’s role in reaction rates and influencing factors.
- Use the Arrhenius equation to describe temperature’s impact on reaction kinetics.
- Explain collision theory’s principles and requirements for effective collisions.
- Outline experimental methods to determine reaction order using rate laws.
- How can you experimentally determine reaction order through initial rate methods or integrated rate laws?
Proudly powered by SirBikramSutradhar
Conclusion
“Some Basic Concepts of Chemistry” is a cornerstone for mastering physical chemistry and succeeding in NEET 2025. Advanced practice questions reinforce your understanding, build confidence, and prepare you to tackle exam challenges effectively. By consistently practicing and revising, you’ll develop a solid foundation to excel in this critical subject. Stay focused, practice diligently, and achieve your goals with determination and perseverance.
NEET (National Eligibility cum Entrance Test):
-
📘 Becoming an Astronaut in India (ISRO) – A Complete Guide After Class 12
📘 Becoming an Astronaut in India (ISRO) – A Complete Guide After Class 12 ✍️…
-
Top 20 Shubh Tracks You MUST Watch on @SHUBHWORLDWIDE 🎧
Top 20 Shubh Tracks You MUST Watch on @SHUBHWORLDWIDE 🎧 📝 Top 20 Shubh Tracks…
-
🌟🎉 Anupa Datta Shines Again with 475 Marks in TBSE Class 12 Science 2025 – A True Inspiration! Tbse Board Result 2025 🎉🌟
📅 Published on: April 30, 2025📍 Agartala, Tripura In a world where challenges often dim…
-
🚀 bAstronautWay – Your Ultimate Learning Destination! 🎓✨
TBSE CBSE Undergraduate and Postgraduate Entrance Exams 📍 Our Locations: 9863002294 / 7005561197 📚 Courses…
-
🏆 Pratik Waikar: The Unstoppable Kho Kho World Cup Captain 2025
Discover the inspiring journey of Pratik Waikar, India’s Kho Kho sensation, as he rises to…
-
🏆Sandeep Arya: The Iron Will of Surya Namaskar
In the world of yoga endurance, one name stands with extraordinary strength and discipline —…
-
Rosan Kujur: Odisha’s Midfield Dynamo Rising for India 🇮🇳🏑
In the modern era of Indian hockey, where speed, tactical intelligence, and composure define greatness,…
-
📘 Becoming an Astronaut in India (ISRO) – A Complete Guide After Class 12

📘 Becoming an Astronaut in India (ISRO) – A Complete Guide After Class 12 ✍️…
-
Top 20 Shubh Tracks You MUST Watch on @SHUBHWORLDWIDE 🎧

Top 20 Shubh Tracks You MUST Watch on @SHUBHWORLDWIDE 🎧 📝 Top 20 Shubh Tracks…
-
🌟🎉 Anupa Datta Shines Again with 475 Marks in TBSE Class 12 Science 2025 – A True Inspiration! Tbse Board Result 2025 🎉🌟

📅 Published on: April 30, 2025📍 Agartala, Tripura In a world where challenges often dim…
-
🚀 bAstronautWay – Your Ultimate Learning Destination! 🎓✨

TBSE CBSE Undergraduate and Postgraduate Entrance Exams 📍 Our Locations: 9863002294 / 7005561197 📚 Courses…
-
🏆 Pratik Waikar: The Unstoppable Kho Kho World Cup Captain 2025

Discover the inspiring journey of Pratik Waikar, India’s Kho Kho sensation, as he rises to…
-
🏆Sandeep Arya: The Iron Will of Surya Namaskar

In the world of yoga endurance, one name stands with extraordinary strength and discipline —…
-
Rosan Kujur: Odisha’s Midfield Dynamo Rising for India 🇮🇳🏑

In the modern era of Indian hockey, where speed, tactical intelligence, and composure define greatness,…
-
⚡ Abinaya Rajarajan – The Fastest Rising Star of Indian Women’s Sprinting 🇮🇳🔥

In the electrifying world of Indian athletics, one name is blazing down the track with…
-
🎓 DEEET 2026: Complete Guide to Tripura Diploma Engineering Admission

DEEET The DEEET 2026 (Diploma Engineering Entrance Examination, Tripura) is the official gateway for students…
-
🏆Dilip Ratan Khandavi – The Relentless Dynamo of Indian Kho-Kho 🇮🇳

In the electrifying world of modern Kho-Kho, where speed meets strategy and endurance defines champions,…
-
🏆Aditya Ganpule – India’s Defensive Wall in Modern Kho Kho

In the fast-evolving world of professional Kho Kho, one name has emerged as a symbol…
-
🌊 Rishika Bodele – The Golden Wave of Indian Swimming & A Trailblazer Beyond the Pool 🇮🇳🏆

In the dynamic world of Indian swimming, Rishika Bodele stands as a symbol of discipline,…
-
🥊Ankit Khatana – India’s Fearless Ring Warrior

🇮🇳 Introduction In the fiercely competitive world of Indian boxing, Ankit Khatana has emerged as…
-
Jeevan S Rathod – India’s Rising Star of Kho Kho

In the fast-paced world of Indian Kho Kho, Jeevan S Rathod is emerging as a…
-
Rajesh Kumar – From Tea Seller to Professional Boxing Warrior

Rajesh Kumar, popularly known in the fighting world as Rajesh Kasana Lukka, is a professional…
-
Akash Togare – The Silent Wall of Ultimate Kho Kho
A Defensive Warrior of the Telugu Yoddhas 🔥 Akash Togare — Rising Defender in Ultimate…
-
Akshay Ganpule – Indian Kho Kho Star & Best Ultimate Kho Kho League Leader
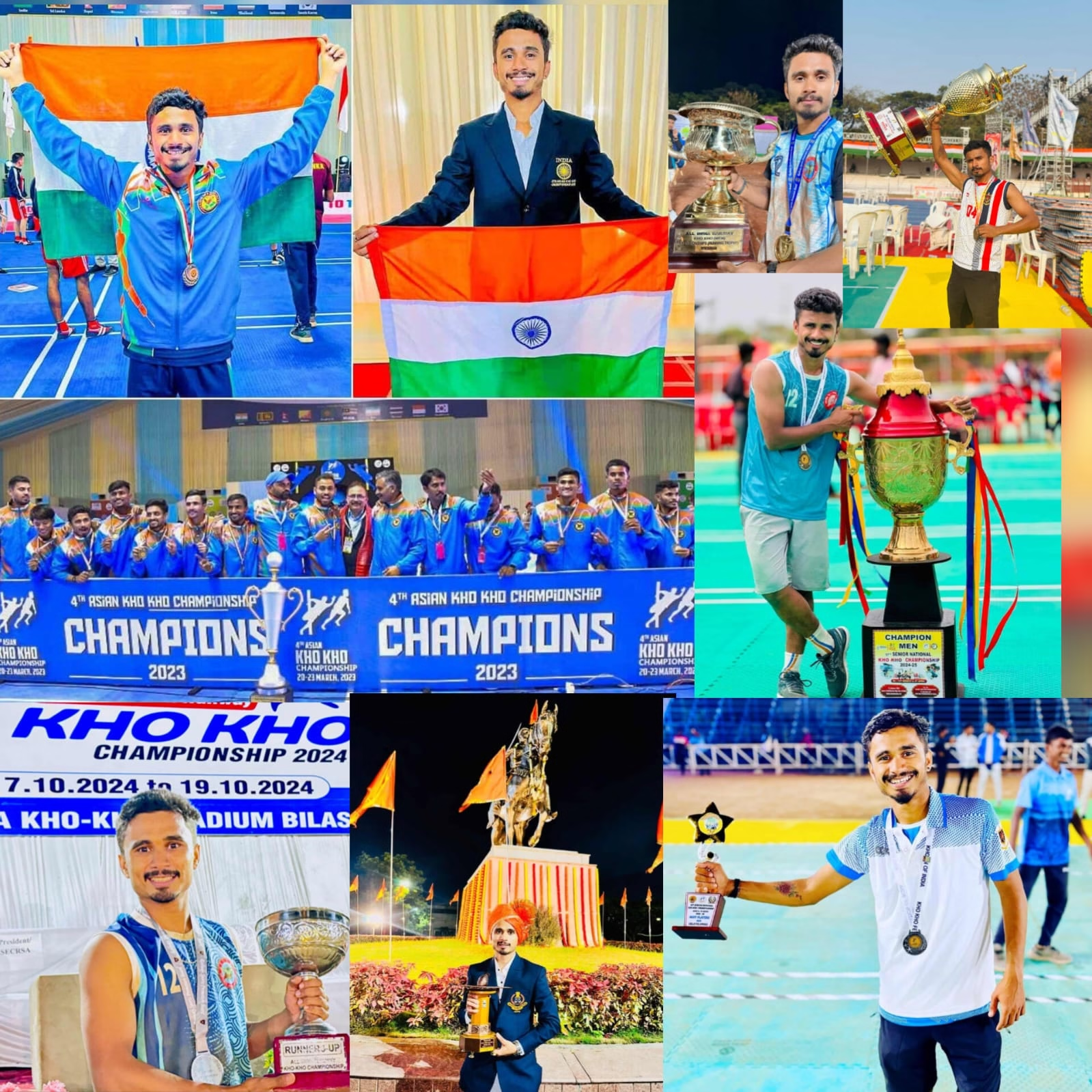
Akshay Ganpule – Indian Kho Kho Star & Best Ultimate Kho Kho League Leader 🏆…
-
Ravi Kumar Punia — A Football Warrior’s Journey From Pitch to Podium

Ravi Kumar Punia : Life, Career & Legacy Introduction Ravi Kumar Punia is an Indian…
-
🇮🇳 Golden Glory for Indian Army at 36th Senior National Canoe Sprint Championship 🇮🇳

🇮🇳 Golden Glory for Indian Army at 36th Senior National Canoe Sprint Championship 🇮🇳 🥇…
-
#1 Ramji Kashyap: The Unstoppable Kho Kho Champion!

#1 Ramji Kashyap: The Unstoppable Kho Kho Champion! From Velapur to World Glory: Ramji Kashyap…
-
🏸🔥Mithun Manjunath – The Unbreakable Rise of an Indian Badminton Warrior 🇮🇳

Mithun Manjunath 🏸🔥 Mithun Manjunath – The Unbreakable Rise of an Indian Badminton Warrior 🇮🇳…
-
🏆 Sreejesh S – India’s Kho Kho Powerhouse Dominating the Ultimate League

🏆 Sreejesh S – India’s Kho Kho Powerhouse Dominating the Ultimate League Verified Athlete Success…
-
🌟 From Adversity to Triumph: The Unstoppable Journey of Amir Hussain Lone

🌟 From Adversity to Triumph: The Unstoppable Journey of Amir Hussain Lone India’s Armless Cricketer…
-
🏅IRON WILL AT 19: Renee Noronha — India’s Youngest Female Ironman Who Redefined Limits 💪🔥
🏅 IRON WILL AT 19: Renee Noronha — India’s Youngest Female Ironman Who Redefined Limits…





