Is Matter Around Us Pure? – CBSE Class 9 Science Chapter 2 Explained with Notes, Diagrams, MCQs & FAQs | By Grandmaster Bikram Sutradhar

Is Matter Around Us Pure? – CBSE Class 9 Science Chapter 2 Explained with Notes, Diagrams, MCQs & FAQs | By Grandmaster Bikram Sutradhar
🏷️
SirBikramSutradhar
📄
Master CBSE Class 9 Science Chapter 2: Is Matter Around Us Pure? with this expert guide by Grandmaster Bikram Sutradhar (SirBikramSutradhar). Explore easy-to-understand notes, definitions, differences, types of mixtures, separation techniques, Tyndall effect, MCQs, and HOTS questions. Includes clear diagrams, real-life examples, FAQs, and revision mind maps—perfect for scoring high in school exams and Olympiads.
❓Top Student FAQs – CBSE Class 9 Chapter 2: Is Matter Around Us Pure?
Q1. What is the meaning of pure substance in this chapter?
A pure substance is made up of only one kind of particle and has consistent properties throughout. Examples: distilled water, gold, oxygen.
Q2. What is the difference between a mixture and a compound?
- Mixture: Components can be separated physically, no fixed ratio.
- Compound: Chemically combined in a fixed ratio, cannot be separated by physical methods.
Q3. What are the types of mixtures?
There are two main types:
- Homogeneous mixture (uniform throughout) – e.g., salt in water
- Heterogeneous mixture (non-uniform) – e.g., oil in water
Q4. What is the Tyndall Effect?
Tyndall effect is the scattering of light by colloidal particles. It helps us distinguish between a true solution and a colloid.
Q6. What are examples of colloids in daily life?
- Milk (liquid in liquid)
- Smoke (solid in gas)
- Foam (gas in liquid)
IS MATTER AROUND US PURE?
Questions set 1

-

9 Powerful Achievements of Sandeep Kumar: The Fearless Journey of India’s Race Walking Warrior
-

11 Inspiring Facts About Siddharth Choudhary – Who Is India’s Rising Shot Put Record Holder
-

15 Inspiring Achievements of Pooja Kadian – India’s First Woman Wushu World Champion Who Created History
-

10 Powerful Lessons from the Inspiring Journey of Yogesh Gamish Pawara – From Village Dreams to Rising Kho Kho Star

















![Is Matter Around Us Pure? – CBSE Class 9 Science Chapter 2 Explained with Notes, Diagrams, MCQs & FAQs | By Grandmaster Bikram Sutradhar 27]() Substances are either elements (like iron, oxygen)or compounds (like water, sugar),
Substances are either elements (like iron, oxygen)or compounds (like water, sugar),
and they cannot be separated by physical methods.
Substance वह पदार्थ है जिसकी रचना और गुण एक जैसे होते हैं।
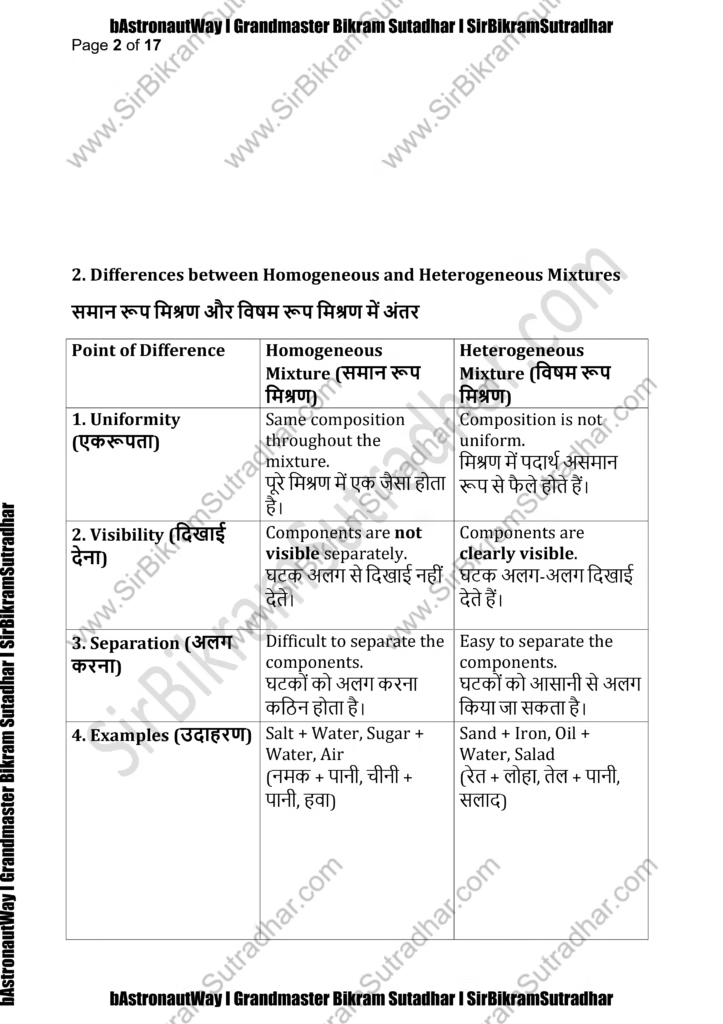
2. Differences between Homogeneous and Heterogeneous Mixtures
समान रूप मिश्रण और विषम रूप मिश्रण में अंतर
Questions set 2
1. Difference between Homogeneous and Heterogeneous Mixtures:
Homogeneous Mixture:
- .
- The particles in a homogeneous mixture are uniformly mixed at the molecular level.
Heterogeneous Mixture:
- The individual components can be seen and distinguished easily.
- The components in a heterogeneous mixture can settle or separate over time.
- Example: Sand in water, a salad, or oil and water mixture.
होमोजीनियस मिश्रण:
- होमोजीनियस मिश्रण का सम्पूर्ण संघटन समान होता है, यानी इसके घटक समान रूप से वितरित होते हैं और इन्हें आसानी से पहचाना नहीं जा सकता।
- होमोजीनियस मिश्रण में कण आणविक स्तर पर समान रूप से मिश्रित होते हैं।
- उदाहरण: पानी में घुला नमक, हवा, या सिरका।
हेटेरोजीनियस मिश्रण:
- हेटेरोजीनियस मिश्रण में दो या दो से अधिक घटक होते हैं, जो समान रूप से वितरित नहीं होते। इसके घटक आसानी से देखे और पहचाने जा सकते हैं।
- हेटेरोजीनियस मिश्रण के घटक समय के साथ बैठ सकते हैं या अलग हो सकते हैं।
- उदाहरण: पानी में बालू, सलाद, या तेल और पानी का मिश्रण।
A sol is a type of colloidal mixture in which solid particles are dispersed in a liquid. The particles are intermediate in size between those in a solution and those in a suspension.
- The particles in a sol do not settle down and cannot be seen easily.
- Example: Ink, paint.
Solution:
A solution is a homogeneous mixture where one substance (solute) dissolves completely in another (solvent). The solute particles are so small that they cannot be seen and do not settle down.
Example: Salt dissolved in water, sugar in tea.
Written By
Full Stack Developer and 5-Time World Record Holder, Grandmaster Bikram Sutradhar
bAstronautWay
SirBikramSutradhar on YouTube
More Story click the link
ICSE CLASS 10 ICSE CLASS 10 BIOLOGY BASTRONAUTWAY SirBikramSutradhar Bikram Sutradhar GrandMaster Bikram Sutradhar selina biology solutions ICSE Biology Selina Solution
class 10 Maths ncert solution.
NCERT Solutions
CBSE Class 10 Mathematics (2025) NCERT Syllabus:
CBSE Class 10 Mathematics NCERT Syllabus (2025) – Chapter-Wise List
CBSE Class 10 Mathematics NCERT Syllabus (2025) – Chapter-Wise List
chapter 4. Quadratic Equations
chapter 5. Arithmetic Progressions
chapter 6. Triangles
chapter 7. Coordinate Geometry
chapter 8. Introduction to Trigonometry
chapter 9. Some Applications of Trigonometry
chapter 11. Areas Related to Circles
chapter 12. Surface Areas and Volumes
class 10 science ncert solution
CBSE Class 10 Science (2025) NCERT Syllabus:
CBSE Class 10 Science NCERT Syllabus (2025) – Chapter-Wise List
CBSE Class 10 Science NCERT Syllabus (2025) – Chapter-Wise List
Chapter 1 Chemical Reactions and Equations
Chapter 2 Acids, Bases and Salts
Chapter 3 Metals and Non-metals
Chapter 4 Carbon and its Compounds
Chapter 5 Life Processes
Chapter 6 Control and Coordination
Chapter 7 How do Organisms Reproduce?
Chapter 8 Heredity
Chapter 9 Light – Reflection and Refraction
Chapter 10 The Human Eye and the Colourful World
Chapter 11 Electricity
Chapter 12 Magnetic Effects of Electric Current
Chapter 13 Our Environment
📖 Chapter 12 – Organic Chemistry (Selina Textbook)
Table of Contents:
- 12A. Organic Compounds
- 12B. Hydrocarbons: Alkanes
- 12C. Hydrocarbons: Alkenes
- 12D. Hydrocarbons: Alkynes
- 12E. Alcohols
- 12F. Carboxylic Acids
- Exercise 12 MISCELLANEOUS
- Glossary
- Model Question Paper –
📌 Exercise 12 MISCELLANEOUS – Focus Areas:
- 🧬 Consolidated practice from the entire Organic Chemistry chapter
- 🧪 Application-based questions covering alkanes, alkenes, alkynes, alcohols, and carboxylic acids
- 🧾 Writing structural formulas and chemical equations
- 💡 Distinguishing reactions, isomer identification, and conversions
- 📘 Full-syllabus revision to boost conceptual clarity and board exam readiness
Each question in this section is designed to:
- ✅ Strengthen cross-topic understanding
- 📝 Prepare students for application-based and reasoning-based questions
- 🧠 Improve problem-solving speed and exam performance







5 thoughts on “Is Matter Around Us Pure? – CBSE Class 9 Science Chapter 2 Explained with Notes, Diagrams, MCQs & FAQs | By Grandmaster Bikram Sutradhar”